
Mucopolysaccharidosis (MPS) IV, or Morquio syndrome, is a progressive, multisystemic lysosomal storage disorder resulting from a deficiency of the enzymes N-acetylgalactosamine 6-sulfatase or β-galactosidase that are responsible for the catabolism of glycosaminoglycans (GAGs),2 which are involved in the building of bones, cartilage, skin, tendons and many other tissues in the body.3 Two distinct forms are recognised: type A (MPS IVA, Morquio A) and type B (MPS IVB). These types differ in the genetic cause of disease, but signs and symptoms among these forms can be similar and present across the spectrum of disease progression.4
MPS IV is progressive, systemic, and lifelong, and it can lead to systemic morbidities and a shortened lifespan.1
Perceived disease rarity, heterogeneous presentation and variability in disease progression make diagnosis challenging and early intervention critical.1,2 Regardless of phenotype, symptoms can progress into end organ damage. Risk factors for increased morbidity include the following4,5:
The majority of patients with Morquio A do not survive past the second decade of life, with frequent causes of death including respiratory failure, complications from surgery and cardiac failure.1
Patients with MPS IV, which is commonly perceived as a musculoskeletal condition,1 can present with or develop unpredictable and clinically heterogeneous symptomatology extending well beyond the obvious manifestations.6 The table below illustrates the potential signs and symptoms of Morquio A, which may be observed either alone or in combination with others, and should raise your suspicion of Morquio A.
Progressive, systemic manifestations can lead to potentially severe cardiovascular,1,7-9 pulmonary,1,6,7,10,11 neurological,6,12 musculoskeletal,1,6 rheumatological,5,13 opthalmalogical,1,14,15 ENT,1,16 hepatic/abdominal13 and dental6,17 consequences. In contrast with other MPS disorders, however, patients with MPS IV do not present with cognitive impairment.
Of note, over 70% of patients manifest with unusual skeletal features within the first 2to 3 years of life.6 Recent research has indicated that approximately 25% of patients with Morquio A syndrome present with a non-classical phenotype.5
Patients can present with classical or non-classical patterns of signs and symptoms. Patients with non-classical phenotype are often reported to have significantly delayed time to diagnosis relative to symptom manifestation.1
Variable disease progression in patients with Morquio A18

Regardless of phenotype,1 symptoms can progress into end-stage organ damage.
Underappreciation of non-classical presentation in Morquio A can lead to delayed or missed diagnoses, with significant impacts:
Surgical considerations
Surgical need and burden is high among patients with Morquio A. According to a natural history study of Morquio A, 70% of the population (mean age 14.5 years) had at least 1 surgical procedure.7
A patient’s surgical history and need should alert you to the possibility of MPS IV.
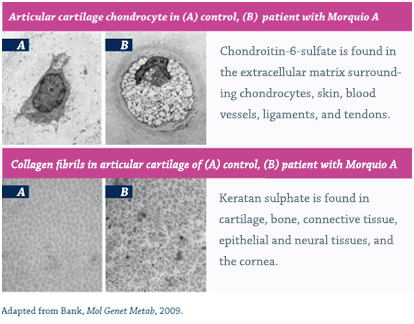
Morquio A and MPS IVB are caused by mutations in the GALNS and GLB1 genes, which encode the enzymes N-acetylgalactosamine 6-sulfatase and β-galactosidase, respectively.1,25 The resulting enzyme deficiencies lead to multiple metabolic pathologies including, most notably, the accumulation of the GAG substrates keratin sulfate and chondroitin-6-sulfate in lysosomes throughout the body.1,26
As lysosomes accumulate, they occupy an increasingly greater area of the cytoplasm, obscuring other organelles and disrupting function.27 The defective enzyme activity in MPS IV leads to cell, tissue and organ system dysfunction that results in the progressive multisystemic morbidities that are the hallmark of this disorder.1,6
Ongoing research is transforming patient management:
Published in 2014, the “International Guidelines for the Management and Treatment of Morquio A Syndrome” establish the standard of care for Morquio A. Due to the progressive nature of the disease, these guidelines urge early initiation of treatment with ERT.
In addition to ERT, ongoing lifetime management and procedural care from a multidisciplinary coordinated care team are critical components to optimising patient outcomes.6 Supportive care includes both medications and surgical interventions, including the following1,7:
Due to the unpredictable, multisystemic nature of MPS IVA, regular, multidisciplinary, comprehensive evaluation and treatment from a coordinated care team is also essential to identify signs of organ damage and ensure optimal patient outcomes.1,6 Doctors can optimise their management by creating a personalised management plan for each of their patients, starting with a thorough assessment schedule for each body system affected by MPS IVA.1
Once diagnosis is confirmed, baseline assessments should be performed, and appropriate management should be initiated.1,26 Ongoing comprehensive assessments, symptomatic treatment and surgical interventions,1,7 continuity of care from the paediatric to the adult setting is a critical consideration for patients and families living with MPS.1,31
Patients with MPS IV often require surgical intervention to address the multisystemic complications of the disease.7 This surgical care is complicated by the nature of the disease.
Patients with MPS IV suffer from multiple factors that can dramatically increase surgical risk and the need for monitoring32:
These factors complicate surgical and anaesthetic care, require pre-planning, and necessitate disease-specific techniques to increase optimal outcomes.33
Specialised perioperative procedures during anaesthesia, such as intubation and extubation, and the use of an intraoperative neuromonitoring checklist, are essential to successful surgical interventions.1,33 An integrated surgical team consisting of MPS VI specialists is crucial for positive, durable outcomes.1
As patients with MPS IV reach adulthood, their relationship with their medical team will change. To help manage this transition, individual plans are necessary to minimise treatment interruptions, extend support beyond the scope of paediatric care and parental support, and ensure that adult patients are knowledgeable in managing MPS IV.1,31
These transition plans should be tailored to each patient’s specific needs, so that those who can take over their own care will have the necessary tools, and those who are more limited will have the appropriate care and services in place to support them. The plans should include an assessment to determine the patients capacity to successfully achieve his or her outlined goals, as well as his or her knowledge and ability to communicate information about his or her condition.31
References: 1. Hendriksz CJ, Berger KI, Giugliani R, et al. International guidelines for the management and treatment of Morquio A syndrome. Am J Med Genet Part A. 2014;9999A:1-15. doi:10.1002/ajmg.a.36833. 2. Northover H, Cowie RA, Wraith JE. Mucopolysaccharidosis type IVA (Morquio syndrome): a clinical review. J Inherit Metab Dis. 1996;19(3):357-365. 3. Islam T, Linhardt RJ. Chemistry, biochemistry, and pharmaceutical potentials of glycosaminoglycans and related saccharides. In: Wong C-H, ed. Carbohydrate-based Drug Discovery. Weinheim, Germany: WILEY-VCH Verlag GmbH & Co KGaA; 2003:407-439. 4. Tomatsu S, Montaño AM, Nishioka T, et al. Mutation and polymorphism spectrum of the GALNS gene in mucopolysaccharidosis IVA (Morquio A). Hum Mutat. 2005;26(6):500-512. 5. Montaño AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis. 2007;30(2):165-174. doi:10.1007/s10545-007-0529-7. 6. Tomatsu S, Montaño AM, Oikawa H, et al. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment: a special review. Curr Pharm Biotechnol. 2011;12(6):931-945. doi:1389-2010/11. 7. Harmatz P, Mengel KE, Giugliani R, et al. The Morquio A clinical assessment program: baseline results illustrating progressive, multisystemic clinical impairments in Morquio A subjects. Mol Genet Metab. 2013;109(1):54-61. doi:10.1016/j.ymgme.2013.01.021. 8. John RM, Hunter D, Swanton RH. Echocardiographic abnormalities in type IV mucopolysaccharidosis. Arch Dis Child. 1990;65(7):746-749. 9. Ireland MA, Rowlands DB. Mucopolysaccharidosis type IV as a cause of mitral stenosis in an adult. Br Heart J. 1981;46(1):113-115. 10. Semenza GL, Pyeritz RE. Respiratory complications of mucopolysaccharide storage disorders. Medicine. 1988;67(4):209-219. 11. Pelley CJ, Kwo J, Hess DR. Tracheomalacia in an adult with respiratory failure and Morquio syndrome. Respir Care. 2007;52(3):278-282. 12. Gulati MS, Agin MA. Morquio syndrome: a rehabilitation perspective. J Spinal Cord Med. 1996;19(1):12-16. doi:10.1159/000202621. 13. Holzgreve W, Gröbe H, von Figura K, Kresse H, Beck H, Mattei JF. Morquio syndrome: clinical findings in 11 patients with MPS IVA and 2 patients MPS IVB. Hum Genet. 1981;57(4):360-365. 14. Danes BS. Corneal clouding in the genetic mucopolysaccharidoses: a cell culture study. Clin Genet. 1973;4(1):1-7. 15. Leslie T, Siddiqui MAR, Aitken DA, Kirkness CM, Lee WR, Fern AI. Morquio syndrome: electron microscopic findings [letter]. Br J Ophthalmol. 2005;89(7):917-929. doi:10.1136/bjo.2004.055400. 16. Hendriksz CJ, Harmatz P, Beck M, et al. Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA. Mol Genet Metab. 2013;110:54-64. doi:10.1016/j.ymgme.2013.04.002. 17. Kinirons MJ, Nelson J. Dental findings in mucopolysaccharidosis type IV A (Morquio's disease type A). Oral Surg Oral Med Oral Pathol. 1990;70(2):176-179. 18. Data on file. BioMarin Pharmaceutical Inc. 19. Berger KI, Fagondes SC, Giugliani R, et al. Respiratory and sleep disorders in mucopolysaccharidosis. J Inherit Metab Dis. 2013;36(2):201-210. doi:10.1007/s10545-012-9555-1. 20. Hendriksz C. Improved diagnostic procedures in attenuated mucopolysaccharidosis. Br J Hosp Med. 2011;72(2):91-95. 21. Clarke LA. Pathogenesis of skeletal and connective tissue involvement in the mucopolysaccharidoses: glycosaminoglycan storage is merely the instigator. Rheumatology (Oxford). 2011;50(suppl 5):v13-18. doi:10.1093/rheumatology/ker395. 22. Lehman TJA, Miller N, Norquist B, Underhill L, Keutzer J. Diagnosis of the mucopolysaccharidoses. Rheumatology. 2011;50(suppl 5):v41-v48. 23. Morishita K, Petty RE. Musculoskeletal manifestations of mucopolysaccharidoses. Rheumatology. 2011;50(suppl 5):v19-v25. doi:10.1093/rheumatology/ker397. 24. Muenzer J. Overview of the mucopolysaccharidoses. Rheumatology (Oxford). 2011;50(suppl 5):v4-v12. doi:10.1093/rheumatology/ker394. 25. Wood TC, Harvey K, Beck M, et al. Diagnosing mucopolysaccharidosis IVA. J Inherit Metab Dis. 2013;36(2):293-307. doi:10.1007/s10545-013-9587-1. 26. VIMIZIM [package insert]. Novato, CA: BioMarin Pharmaceutical Inc; 2014. 27. Coutinho MF, Lacerda L, Alves S. Glycosaminoglycan storage disorders: a review. Biochem Res Int. 2012;2012:471325. doi:10.1155/2012/471325. 28. Bank RA, Groener JEM, van Gemund JJ, et al. Deficiency in N-acetylgalactosamine-6-sulfate sulfatase results in collagen perturbations in cartilage of Morquio syndrome A patients. Mol Genet Metab. 2009;97(3):196-201. doi:10.1016/j.ymgme.2009.03.008. 29. Monzon ME, Casalino-Matsuda SM, Forteza RM. Identification of glycosaminoglycans in human airway secretions. Am J Respir Cell Mol Biol. 2006;34(2):135-141. doi:10.1165/rcmb.2005-0256OC. 30. Harmatz P, Mengel KE, Giugliani R, et al. Longitudinal analysis of endurance and respiratory function from a natural history study of Morquio A syndrome. Mol Genet Metab. 2015;114(2):186-194. doi:10.1016/j.ymgme.2014.10.015. 31. American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians, Transitions Clinical Report Authoring Group, Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128(1):182-200. doi:10.1542/peds.2011-0969. 32. Walker R, Belani KG, Braunlin EA, et al. Anaesthesia and airway management in mucopolysaccharidosis. J Inherit Metab Dis. 2013;36(2):211-219. doi:10.1007/s10545-012-9563-1. 33. Theroux MC, Nerker T, Ditro C, Mackenzie WG. Anesthetic care and perioperative complications of children with Morquio syndrome. Paediatr Anaesth. 2012;22(9):901-907. doi:10.1111/j.1460-9592.2012.03904.x.